Leishmaniasis
Leishmaniasis refers to a diverse group of diseases, all caused by single-celled parasites called Leishmania. up to 350 million people worldwide are at risk from leishmaniasis and about 12 million are infected at any one time. There are between 900 thousand to 1.6 million new cases of leishmaniasis each year causing 20,000-30,000 deaths annually. The disease is recognized by the World Health Organisation (WHO) as a major neglected disease of poverty that disproportionately affects populations in low and middle-income countries.
Watch our video "A few thing you might not know about leishmaniasis"
https://www.youtube.com/embed/DgchaulbtrY

Sandfly that spreads Leishmaniasis (credit: CDC)
Where is Leishmaniasis found?
Leishmaniasis is found in 98 countries worldwide. The majority of cases of visceral leishmaniasis are found in five countries (India, Nepal, Bangladesh, Sudan and Brazil), whereas cutaneous leishmaniasis is found in most parts of the Middle East as well as South, Central and Latin America. Although the major impact of the disease is found in developing countries, leishmaniasis transmission also occurs in European countries bordering the Mediterranean, where clinical disease is often associated with immune deficiency (e.g. due to HIV infection). Leishmaniasis in refugees fleeing conflict is an increasing problem, although in the absence of the sand fly vector, they are unlikely to spread disease in northern Europe. Leishmaniasis has been reported in the US, likely due to the northern spread of sand flies from Central and Latin America.
How is Leishmaniasis spread?
Leishmaniasis is spread by the bite of female sand flies. Sand flies are much smaller than mosquitoes, making bed nets less effective for preventing leishmaniasis than malaria.
What are the symptoms of Leishmaniasis?
The symptoms of leishmaniasis are variable, and depend on the type of parasite you are infected with. In some forms of leishmaniasis, a nodule develops at the site of the sand fly bite and this may or may not ulcerate into an open lesion with a crater-like appearance. This is often referred to as simple cutaneous leishmaniasis and the lesions typically self-heal after several months, leaving a scar. If the lesion is on soft tissue, e.g. the ear lobe, there may be significant tissue destruction.
In some cases, parasites may spread from the primary lesion and invade the mucosal tissues of the mouth, upper palate and nose. Although the primary skin lesion may heal successfully, mucosal tissue may be significantly damaged, leading to disfigurement and difficulty in eating. This form of disease is called mucosal or mucocutaneous leishmaniasis. Collectively, these different types of cutaneous leishmaniasis impact quality of life for millions of people.
Some Leishmania parasites are able to invade deep tissues like the spleen, liver and bone marrow causing visceral leishmaniasis or kala azar. This is usually fatal if not treated and visceral leishmaniasis results in 20,000-40,000 deaths annually, making it the second most important parasitic disease in terms of mortality after malaria. Some patients who are successfully treated for visceral leishmaniasis may go on to develop a chronic skin disease with nodules, papules or hypo-pigmented macules on the face and upper body (called post kala azar dermal leishmaniasis or PKDL).
Is Leishmaniasis curable?
Yes, but treatment is often long, painful and may have serious side effects, particularly when started late. AmbiSome has revolutionised the treatment of visceral leishmanaisis in South Asia, but it is less effective elsewhere. Treatment for cutaneous leishmaniasis has changed little in >50 years. Like other microbes, the Leishmania parasites can develop drug resistance.
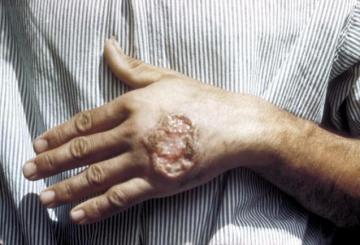
Skin ulcer caused by Leishmaniasis (credit: CDC)
Vaccines against Leishmaniasis
There is no vaccine against human leishmaniasis at the moment. A veterinary vaccine is available to protect dogs from contracting visceral leishmaniasis.
VALIDATE
At the VALIDATE Network, by bringing together Leishmaniasis researchers from around the world we hope to speed up progress towards developing vaccines against this disease.
Further Information:
WHO Leishmaniasis factsheet:
WHO endemicity maps:
Videos about Leishmaniasis:
Leishmania are protozoan parasites that are transmitted between mammalian hosts by biting Phlebotomine sand flies. Approx. 20 species of Leishmania infect humans and cause a spectrum of clinical diseases that affect the skin and mucosa (tegumentary leishmaniasis) or systemic tissues (visceral leishmaniasis). Although the type of leishmaniasis is often closely associated with parasite species, this is not always the case, and varying clinical presentations may occur e.g. due to malnutrition or host genetic diversity.
Found in 98 countries worldwide, around 12 million people are infected with leishmaniasis at any one time, causing 20,000-40,000 deaths annually [1,2]. Most forms of leishmaniasis are zoonotic diseases, infecting a range of hosts including rodents and canids, but in some cases transmission may be restricted to humans (anthroponotic VL). Canine VL is an important veterinary disease and an important target for controlling disease in humans.
Importantly, many people exposed to these parasites do not develop clinical leishmaniasis, indicating the ability of the human immune system to control infection in most cases. However, suppression of the immune system by co-infection with human immunodeficiency virus (HIV) or by elective therapy (e.g. for autoimmune disease) may lead to clinical leishmaniasis [3]. Drug resistance is of increasing concern in leishmaniasis. Diagnosis of the disease is by looking for the parasites in biopsies, by antibody-based tests or by clinical exam. Developing drugs for leishmaniasis is complicated as these are eukaryotic parasites and share much of their biochemistry with human cells. Most anti-leishmanials, therefore, have narrow therapeutic windows; one contains a heavy metal (antimony), whilst another is teratogenic. The most effective drug, liposomal Amphotericin B (AmbiSome) has revolutionised treatment for VL in South Asia, but is much less effective in other parts of the world. Drugs for CL have changed little in >50 years. Many treatment courses are protracted, painful and have significant side effects. An effective vaccine could revolutionise leishmaniasis control [4,5].
The challenges of developing a leishmaniasis vaccine
Developing an effective vaccine for leishmaniasis is not easy, but epidemiological, clinical and experimental data suggests it should be possible [4,5]. It is known that deliberate infection with live Leishmania can prevent against re-infection (this practice of “leishmanisation” was once widespread in the Middle East) and vaccines have been developed for use in dogs. Vaccines for leishmaniasis could be used to prevent disease (prophylactic vaccines) or to treat patients by stimulating their immune system (therapeutic vaccines).
Some of the scientific challenges in leishmaniasis vaccine development are highlighted below:
Lack of immune correlates
For some vaccines, we know exactly the level of a particular kind of immune response that is needed for the vaccine to work – i.e. to stop someone getting disease. For leishmaniasis, we do not know what kind of immune response is needed, or what level of response is required. This means we can only test if a vaccine works in humans by doing large, expensive and time consuming human efficacy trials. This limits the number of vaccines that can be tested in humans.
Uncertain predictive value of animal models
It is not possible to test a vaccine in humans without testing it first in animals. However we do not know which if any of the animal models best predicts efficacy in humans. Again, this means we can only determine if a vaccine will work in humans by testing it in large, expensive, human efficacy trials.
Importance of the vector
Unlike some diseases, such as TB, where transmission occurs directly between people, leishmaniasis is a vector-borne disease. It is now known that during the taking of a blood meal, sand flies introduce a variety of proteins into the mammalian host that affect local immune function. The effects of these proteins have to be taken into account in developing a vaccine for leishmaniasis. Indeed, some have been proposed as candidates to include in a vaccine [6]!
Difficulty of working with leishmania
Some forms of leishmaniasis (VL, mucocutaneous leishmaniasis) are particularly serious once contracted and difficult to treat, and so in some countries (including the UK) they can only be studied in laboratories using special containment measures (called Category or BSL 3). To study natural transmission of disease, sand flies also need to be reared in captivity and once experimentally infected, these sand flies must be kept under BSL 3 containment to prevent their escape. This limits the number of laboratories that can work on the most serious forms of leishmaniasis.
How VALIDATE is helping
By bringing together researchers working on different (but similar) pathogens, discoveries in one field can be more quickly taken advantage of in research against another pathogen. Bringing together researchers from different disciplines and institutes in new collaborations means knowledge can be exchanged and new research ideas for the field can be generated and investigated. Bringing new researchers into this field, and progressing the careers of early career researchers, will aid with new ideas and the continuation of the field into the future.
Further reading (find more papers on our publications page)
Gillespie PM et al. Status of vaccine research and development of vaccines for leishmaniasis. Vaccine. 2016; 34(26):2992-2995.
References
1. Rio Ribeiro R et al 2018. Canine leishmaniasis: an overview of the current status and strategies for control. Biomed Res Int 2018:3296893.
2. Dantas-Torres F et al 2019. Canine leishmaniasis control in the context of One Health. Emerg Infect Dis 25(12):1-4.
3. Fletcher K, Issa R, Lockwood DN. Visceral leishmaniasis and immunocompromise as a risk factor for the development of visceral leishmaniasis: a changing pattern at the hospital for tropical diseases, london. PloS one. 2015;10(4):e0121418.
4. Alvar J, Croft SL, Kaye P, Khamesipour A, Sundar S, Reed SG. Case study for a vaccine against leishmaniasis. Vaccine. 2013;31 Suppl 2:B244-9.
5. Gillespie PM, Beaumier CM, Strych U, Hayward T, Hotez PJ, Bottazzi ME. Status of vaccine research and development of vaccines for leishmaniasis. Vaccine. 2016;34(26):2992-5.
6. Reed SG, Coler RN, Mondal D, Kamhawi S, Valenzuela JG. Leishmania vaccine development: exploiting the host-vector-parasite interface. Expert review of vaccines. 2016;15(1):81-90.

The effect of BCG vaccination in immune responses against visceral leishmaniasis in a natural (canine) model of infection
Led by Dr Javier Salguero Bodes (Public Heath England, UK), with Dr Isadora dos Santos Lima (FIOCRUZ, Brazil), Assoc Prof Daniela Farias Larangeira (UFBA, Brazil), Dr Deborah Fraga (FIOCRUZ), Dr Geraldo Sá Oliveira (FIOCRUZ), Dr Washington dos-Santos (FIOCRUZ), and Prof Luiz Freitas (FIOCRUZ)
Read more here

Identification of Leishmania donovani and Mycobacterium tuberculosis- derived proteins on the surface of infected macrophages that are associated with ADCC induction
Led by Dr Mohamed Osman (University of York, UK), with Prof Paul Kaye (University of York, UK), Dr John Pearl (University of Leicester, UK) and Prof Andrea Cooper (University of Leicester, UK)
Read more here

Protective efficacy of conserved Leishmania hypothetical proteins against visceral leishmaniasis
Led by Prof Myron Christodoulides (University of Southampton, UK), with Assoc Prof Eduardo Coelho(Federal Unversity of Minas Gerais, Brazil)
Read more here

Cytomegalovirus as a risk factor for TB and leishmaniasis
Led by Dr Lisa Stockdale (University of Oxford, UK), with Dr Robindra Basu Roy (LSHTM, UK), Dr Vivian Tamietti Martins (Federal University of Minas Gerais, Brazil), Assoc Prof Eduardo Coelho (Federal University of Minas Gerais, Brazil), and Prof Helen Fletcher (LSHTM, UK)
Read more here


